

- [email protected]
+91 40 2344 5962 - View Stock Quote


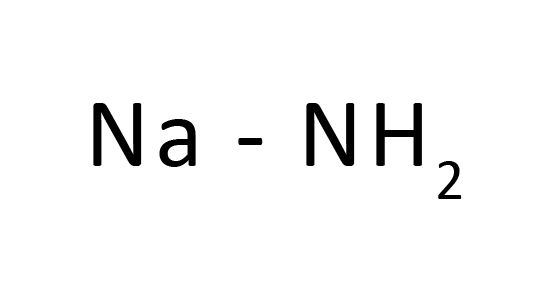

Synonyms : Sodamide
CAS No. : [7782-92-5]
Molecular Formula : NaNH 2
Formula weight : 39.01
Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Sodium amide is mainly used as a strong base in organic chemistry, often in liquid ammonia solution. It is the reagent of choice for the drying of ammonia.
Sodium Amide is a crystalline compound which decomposes explosively in contact with water. It is considered as a strong basic condensing agent and is generally prepared by passing ammonia through molten sodium, and is used chiefly in making sodium cyanide. Sodamide is the common name for sodium amide is salt composed of sodium cations and the azanide anion. The solid, which is dangerously reactive toward water is white but the one which is commercially available are typically grey due to the presence of small quantities of metallic iron during the manufacturing process.
Sodium amide is generally prepared by reacting sodium metal with ammonia gas or liquid ammonia. This is the common production method of production where sodium is reacted with liquid ammonia in the presence of iron nitrate as a catalyst to speed up the process. This reaction further proceeds with the formation of an electride intermediate, which ultimately gives sodium amide and hydrogen gas. The complete procedure is here:
The liquid ammonia is condensed in the flask and powdered ferric nitrate hexahydrate is added to it. The mixture is stirred well and then sodium is introduced to it. The solution is stirred continuously until the blue colour of the solution changes to grey. Sodium amide prepared in this way should never be stored in the dry solid state since these solid forms are explosive compounds.
Sodium amide can also be prepared by passing dry gases like ammonia over sodium at a temperature of 350° C. In this process clean sodium is placed a tube for combustion wherein dry ammonia is passed over it which then heated. The end product obtained is thus stored away from moisture.
Sodium amide has a basic property so, is used in many chemical reactions, especially in organic synthesis. It is also essentially used in the preparation of some dyes such as indigo and several other organic compounds like hydrazine and sodium cyanide.